Better sleep quality.
Client Profile
Sommetrics strives to improve health by helping to achieve better sleep quality.
50-70 million US adults have a sleep disorder, and obstructive sleep apnea is one of the most prevalent sleep disorders. Approximately 3-7% of men and 2-5% of women have sleep apnea. Worldwide, over 100 million people suffer from sleep apnea. The company is developing innovative solutions (technology and services) that help assess and prevent the narrowing of the upper airway. These problems range from sedation-related obstructions (or apneas) to obstructive sleep apnea and snoring.
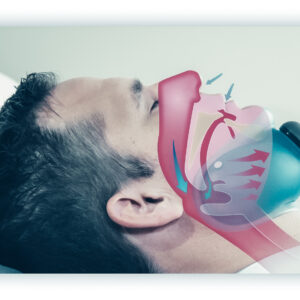
aerSleep™ is the first product for sleep apnea that is completely non-invasive and does not require connecting tubes or retaining straps. aerSleep uses aer+TM technology (formerly known as cNEP), which is its patented technology that uses the application of negative external air pressure on the outside of the neck to gently open the airway.
aerSleep is a simple to use, non-invasive external airway device that effectively and safely reduces the number of apnea and oxygen desaturation events.
The Brief
Sommetrics engaged Extel to undertake a full electronic product design review to include Design for Manufacturing (DFM) and to assist Sommetrics to optimize the performance and reliability of its electronics assemblies, ensuring compliance with ISO 13485 Medical Device guidelines.
Industry
Medical and Healthcare
Location
USA
Involvement
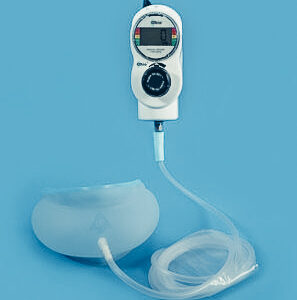
- Design for manufacturing (DFM) review of Sleep Apnea Pump Controller PCB assembly to address design challenges
- Engineering design of Sleep Apnea Pump Controller PCB assembly
- Manufacturing of PCBA Sleep Apnea Pump Controller PCB assembly
Video Testimonial
Project challenges
- Time to market: any uncovered issues would directly impact how soon Sommetrics would be able to launch their products as first to market always carries a competitive advantage
- Cost of goods (COGs): any DFM changes recommended by Extel should not have a significant impact on the cost of components or manufacturing of the products
- Design changes: any DFM design changes recommended by Extel should not have a significant impact on the complexity of the device, however, should increase reliability
Addressing the challenges
- Use of an Integrated Master Schedule: this ensures our engineering group is aligned with Sommetrics milestones that will allow us to deliver the agreed project deliverables on time and on budget.
Core objectives
Sommetrics supplied 23 Sample PCBAs, of which 10 were known to work and the remaining 13 having a combination of the following defects: Bluetooth failure, SCM Connection failure, battery charging failure, USB Power failure, not oscillating. Extel was tasked with uncovering why these issues were present and provide a solution to fix these critical issues.
The Outcome
Our investigation strategy was to first look for manufacturing defects by both optical and x-ray inspection and then consider design and component defects.
All PCBAs were optically inspected under 20 x magnification stereo microscope and a selection were x-rayed using Xaxis equipment. Using these diagnostic quality inspection tools allowed us to identify the manufacturing defects root cause.
In addition to providing all ongoing electronic manufacturing requirements, we also support the design and manufacture of the next gen Sleep Apnea Pump Controller PCB Assembly, as well as various ancillary medical device products for Sommetrics.
We are proud to have become Sommetrics’ design and manufacturing partner and act as an extension of their engineering group.
Business advantages
Sommetrics has gained several advantages as an outcome of this project and our ongoing work with them.
Technical advantages
FDA device designation: as most medical devices are a combination of high complexity, high quality, high reliability and high compliance, it needs to adhere to stringent qualification criteria to gain FDA approval. FDA approval is a significant milestone that aids Sommetrics in getting their device to market and commercial success.
Operational advantages
High volume manufacturing: as this is a medical device that needs 100% uptime in a healthcare or home setting, every PCB manufactured needs a predictable functional level with high quality and high reliability in mind.

Technologies
Optical and 3D x-ray inspection
All PCBs were optically inspected under 20 x magnification stereo microscope and a selection was x-rayed using Xaxis equipment
Partners
ISO 13485 medical device compliance
Strategic or competitive advantages
Sommetrics now has high quality and highly reliable patented aer+™ technology that involves the application of negative external pressure or vacuum to the outside of the neck. This system has advantages over existing treatments for sleep apnea.
Return on investment
The aer+™ technology is also incorporated into the aerFree™ product that is cleared by the FDA for use in the acute care setting
Let’s talk
Speak with one of our team members about how we can best
help your business move forward.
Sign up for our news
Be the first to hear about industry news or Extel updates.